Reduction of 3,4-dihydro-5H-benzo[cd]pyren-5-one.
SyntheticPage 536
DOI:
Submitted: February 9, 2012, published: March 12, 2012
Authors
Anish Mistry (a.mistry@warwick.ac.uk)
A contribution from
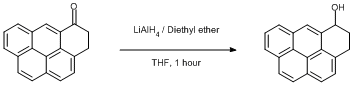
Chemicals
3,4-dihydro-5H-Benzo[cd]pyren-5-one (prepared)
LiAlH4 (1 equiv, Alfa Aesar)
THF
Diethyl ether
LiAlH4 (1 equiv, Alfa Aesar)
THF
Diethyl ether
Procedure
Under a dinitrogen atmosphere, lithium aluminium hydride (3 mg, 0.08 mmol) in diethyl ether was stirred at 0oC. The 3,4-dihydro-5H-Benzo[cd]pyren-5-one (20 mg, 0.08 mmol) was dissolved in THF and added to the mixture slowly and the solution left to stir at room temperature for 1 h.
After 1 hour, water and then HCl was added (1:3) sequentially under a dinitrogen atmosphere.
The organics collected and dried with Na2SO4 and solvent removed under vacuo, to obtain a crude orange solid.
The crude was columned using (8:2) petroleum ether: ethyl acetate to obtain a pinkish powdered solid (19 mg, 94%).
After 1 hour, water and then HCl was added (1:3) sequentially under a dinitrogen atmosphere.
The organics collected and dried with Na2SO4 and solvent removed under vacuo, to obtain a crude orange solid.
The crude was columned using (8:2) petroleum ether: ethyl acetate to obtain a pinkish powdered solid (19 mg, 94%).
Author Comments
Particular care should be taken when quenching the reaction due to any unreacted Lithium Aluminium Hydride.
Data
δH(500MHz, CDCl3) ppm: 2.30 - 2.43 (2H, m, HO-CH2-CH2), 3.37 (1H, ddd, J 5.0, 7.0, 16.5, HO-CH2-CH2), 3.58 (1H, ddd, J 5.0, 8.0, 16.5, HO-CH2-CH2), 5.29 (1H, dd, J 3.5, 7.0, HO-CH), 7.83 (1H, d, J 8.0, aryl), 7.96 - 8.17 (7H, m, aryl).
Lead Reference
Selective Reductions. XII. Explorations in Some Representative Applications of Aluminum Hydride for Selective Reductions,
Nung Min Yoon, Herbert C. Brown, JACS., 90.11, 1968.
Nung Min Yoon, Herbert C. Brown, JACS., 90.11, 1968.
Keywords
ketones, reduction