Coupling of 2,3,5,6-tetrafluoro-4-hydroxy-benzoic acid to aminomethyl polystyrene resin
SyntheticPage 52
DOI:
Submitted: August 15, 2001, published: August 15, 2001
Authors
Daniel Hamza (danielhamza@yahoo.co.uk)
A contribution from
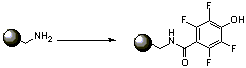
Chemicals
Aminomethyl polystyrene resin (Novabiochem);
2,3,5,6-tetrafluoro-4-hydroxy-benzoic acid hydrate (Aldrich);
1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC, Lancaster);
diisopropylethylamine (DIPEA, Hunig's base, Lancaster);
N,N-dimethylformamide (DMF, Avocado 99%)
2% ninhydrin solution in n-butanol (BDH, TOXIC!)
2,3,5,6-tetrafluoro-4-hydroxy-benzoic acid hydrate (Aldrich);
1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC, Lancaster);
diisopropylethylamine (DIPEA, Hunig's base, Lancaster);
N,N-dimethylformamide (DMF, Avocado 99%)
2% ninhydrin solution in n-butanol (BDH, TOXIC!)
Procedure
1. Coupling of phenol to resin: Add phenol (4.254g, 18.65mmol, 3 eq) to 10mL DMF and dissolve by sonication. Cool in ice water bath and add 4 eq DIPEA (4.33 mL). In separate vessel add 2eq of DIPEA (2.17mL) to EDC (3.575g, 18.65mmol, 3eq) in about 5 mL DMF to give slurry. Add slurry in portions to the phenol solution, it will dissolve as it reacts. Sonicate for about 10 min more for complete dissolution and to allow for active ester/anhydride formation. Meanwhile pre-swell the resin (8.0g, 1 eq, 0.777mmol/g, 6.216 mmol) in minimum volume of DCM and then add the coupling solution and shake overnight adding more DCM if required to give good mixing, but keeping overall concentration as high as possible. Wash resin with DMF, DCM, diethyl ether and THF 3 x 40mL each with mixing. Ninhydrin spot test seems to be the most sensitive way of checking for the complete consumption of amine. Just add a few drops of commercially available 2% ninhydrin solution (HIGHLY TOXIC!) in n-butanol to a few beads of resin in a glass vial and heat carefully in a fume hood using a heat gun. Positive test is purple after a couple of mins. Perform an analogous test on a control resin which should give a negative result (eg chloromethyl). If free amine is still detected, repeat the coupling procedure as above, using 2/3 the amount of each reagent.
2. Cleavage of tetrafluorophenol-tetrafluorobenzoic acid esters: These esters will form due to the phenol coupling to itself. Destroying them is easily performed by suspending the resin in 20% piperidine/DMF for 1h with mixing. Wash resin thoroughly as piperidine sticks to resin. Use DMF, THF, Et2O and DCM as before.
3. Quenching of phenoxide-piperidine salt with HCl: Cleavage of the esters in the previous step leaves behind the piperidine salt and it's important to remove this for accurate loading analysis and to prevent piperidine interference in later reactions. For 6.216mmol of resin, add 6.216mL of 2.0M HCl(aq) in approx 60mL DMF (make up the HCl/DMF solution first) and shake for 2h. Wash well with DCM, DMF, THF, ether and dry in vacuo. Maximum loading in this case would be 0.6761mmol/g. Yield typically: 90-100% after 2 couplings as described above. Assessed as described below.
4. Loading analysis: To 100mg of resin (dry and accurately weighed out), add about 2mL DCM and 10 eq DIPEA then 10eq acetic anhydride, shake overnight. Wash well with DCM, THF, Ether. Cleave with amine of choice, eg 4-methylbenzylamine, 3 eq in DCM, 2mL overnight. Wash resin through onto a silica plug to remove excess amine and vac down to give pure amide product. Assess yield based on the mass of product gained. Typically 90-100%.
2. Cleavage of tetrafluorophenol-tetrafluorobenzoic acid esters: These esters will form due to the phenol coupling to itself. Destroying them is easily performed by suspending the resin in 20% piperidine/DMF for 1h with mixing. Wash resin thoroughly as piperidine sticks to resin. Use DMF, THF, Et2O and DCM as before.
3. Quenching of phenoxide-piperidine salt with HCl: Cleavage of the esters in the previous step leaves behind the piperidine salt and it's important to remove this for accurate loading analysis and to prevent piperidine interference in later reactions. For 6.216mmol of resin, add 6.216mL of 2.0M HCl(aq) in approx 60mL DMF (make up the HCl/DMF solution first) and shake for 2h. Wash well with DCM, DMF, THF, ether and dry in vacuo. Maximum loading in this case would be 0.6761mmol/g. Yield typically: 90-100% after 2 couplings as described above. Assessed as described below.
4. Loading analysis: To 100mg of resin (dry and accurately weighed out), add about 2mL DCM and 10 eq DIPEA then 10eq acetic anhydride, shake overnight. Wash well with DCM, THF, Ether. Cleave with amine of choice, eg 4-methylbenzylamine, 3 eq in DCM, 2mL overnight. Wash resin through onto a silica plug to remove excess amine and vac down to give pure amide product. Assess yield based on the mass of product gained. Typically 90-100%.
Author Comments
1. Coupling step. The procedure has been repeated about 5 times on scales varying from about 10g of resin as above down to 200mg without change in yields. This coupling works very well and probably doesn't require EDC as the coupling agent. However, I would avoid the use of the cheaper DCC since I did encounter problems associated with the removal of DCCU when trying to couple acids to the polymer-supported phenol. An excess of DIPEA is used to help with dissolution of the EDC.HCl and sonication is particularly effective during the preparation of the coupling mixture. 2,3,5,6-tetrafluoro-4-hydroxy-benzoic acid hydrate is expensive (£60 for 5g) and so this is why a greater excess of reagents are not used in the first coupling. Using 3 eq and then 2eq seems to be about the minimum that one can get away with. Any suggestions for a cheap synthetic route to this precursor would be helpful.
2. The esters present after the coupling step can be seen by IR - band at 1765cm-1 - see reference below.
3. Don't use too much HCl in this step to prevent amide bond cleavage. 2 eq works well. This phenol resin is used as a means for amide and sulfonamide library synthesis and so it's important not to have piperidine around to cleave off the esters as they form.
4. Loading analysis requires two chemical steps, but it does give a good idea of what kind of maximum yield one can expect from the resin. SCX cartridges available from Isolute provide an alternative for the removal of excess amine and work extremely well with good functional group selectivity. Quite expensive though (£0.5-£1 each). Final general comments: The reference below also provides good experimental details and some examples of this resin in use. Methanol has not been used as a solvent for washing this resin as this could potentially cause problems in later steps by cleaving the active esters formed (it can often be hard to remove all traces of a solvent/reagent from resin so it's best not to use it at all if it's not necessary). Solid phase glass vessels can be purchased from Radleys or plastic alternatives may be purchased from a company such as Alltech or Whatman.
2. The esters present after the coupling step can be seen by IR - band at 1765cm-1 - see reference below.
3. Don't use too much HCl in this step to prevent amide bond cleavage. 2 eq works well. This phenol resin is used as a means for amide and sulfonamide library synthesis and so it's important not to have piperidine around to cleave off the esters as they form.
4. Loading analysis requires two chemical steps, but it does give a good idea of what kind of maximum yield one can expect from the resin. SCX cartridges available from Isolute provide an alternative for the removal of excess amine and work extremely well with good functional group selectivity. Quite expensive though (£0.5-£1 each). Final general comments: The reference below also provides good experimental details and some examples of this resin in use. Methanol has not been used as a solvent for washing this resin as this could potentially cause problems in later steps by cleaving the active esters formed (it can often be hard to remove all traces of a solvent/reagent from resin so it's best not to use it at all if it's not necessary). Solid phase glass vessels can be purchased from Radleys or plastic alternatives may be purchased from a company such as Alltech or Whatman.
Data
FTIR: After step 1: 1765 and 1656cm-1 After step 3: 1656cm-1 only
Lead Reference
Salvino, J.M.; Kumar, N.V.; Orton, E.; Airey, J.; Kiesow, T.; Crawford, K.; Mathew, R.; Krolikowski, P.; Drew, M.; Engers, D.; Krolikowski, D.; Herpin, T.; Gardyan, M.; McGeehan, G.; Labaudiniere, R. J. Comb. Chem., 2000, 691-697.
Keywords
52, amide, coupling, phenol, resin, solid phase, tetrafluorophenol