Chlorination of 2-phenylcyclopentanone oxime
SyntheticPage 511
DOI:
Submitted: October 4, 2011, published: January 19, 2012
Authors
John Davidson (jsdcrosslaw@tiscali.co.uk)
A contribution from
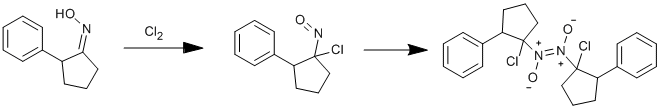
Chemicals
2-phenylcyclopentanone oxime
diethyl ether
chlorine
pentane
alumina
drikold (solid CO2)
acetone
ethanol
benzene
chloroform
Procedure
2-phenylcyclopentanone oxime (0.5 g) was dissolved in diethyl ether (15 ml), the flask, containing the solution, was cooled in ice and a slow stream of chlorine was passed into the solution through a delivery tube until the blue colour, which first formed, changed to green. The excess chlorine and the ether was removed in the fume cupboard in a stream of air and the residue dissolved in pentane, the solution washed with water , separated, and the solution dried over sodium sulphate, then passed through a column of alumina and the blue eluate concentrated and then cooled, with drikold and acetone, to afford a blue solid which was filtered off, placed in an agate mortar , and on warming melted to a blue oil (0.2 g., 34%). After a few days in a refrigerator, the oil solidified to a colourless solid which was then ground to a colourless amorphous solid. It dissolves slowly in benzene, more readily in chloroform.
Author Comments
Very few dimeric gem-chloronitroso compounds have been reported.
Work in subdued light. When solutions of the monomer in ethanol or benzene are exposed to red light a hypsochromic shift of 5 nm and a reduction of εmax was observed. Absorption measurements were consistent with a fairly rapid photomutarotation followed by a much slower photolysis.
Data
Solid began to turn blue at 78°, mp 84°
Found: C 63.23%, H 5.66%, Cl 16.69%, N 6.60% (C11H12ClNO)2 requires: C 63.01%, H 5.77%, Cl 16.91%, N 6.68% ε max 44 at 643 nm (in benzene)
Cryoscopic measurements gave a molecular weight of 205. Calc. for C11H12ClNO: 209.7 and Beer’s Law holds over the range studied (up to 1%)
Lead Reference
Other References
K. A. Ogloblin, T. N. Grigorova, and A. A. Potekhin, Zhurnal Organicheskoi Khimii, 5, 1360-1363 (1969) (English translation 1329-1332)
Mohamed-Cherif Boucenna, John S. Davidson, Anthony McKee, Andrew L. Porte, and David C. Apperley, J. Chem. Soc., Perkin Transactions. 2, 1381-1387 (1995) doi: 10.1039/P29950001381
A. J. N. Hope and Stotherd Mitchell, J. Chem. Soc., 1953, 3483-3486; 1954, 4215-4218
Keywords
addition, dimerisation, oxime, photochemical