Preparaton of the Dess-Martin Periodinane
SyntheticPage 51
DOI:
Submitted: August 15, 2001, published: August 15, 2001
Authors
lisa frost (lisa.frost@evotec.com)
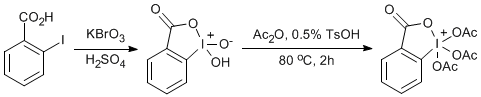
Chemicals
Potassium Bromate
2-Iodobenzoic acid
sulfuric acid
Acetic anhydride
TsOH.H2O
2-Iodobenzoic acid
sulfuric acid
Acetic anhydride
TsOH.H2O
Procedure
Potassium bromate (15.2 g, 91 mmol, 1 equiv) was added over a 30 minute period to a vigorously stirred solution of 2-iodobenzoic acid (17.2 g, 69 mmol, 0.75 equiv) and 14.6 mL of 0.73 M H2SO4. The temperature of the reaction was maintained below 55 oC by the aid of an ice bath. The resulting mixture was warmed to 65 oC and stirred for 4 h. The slurry was cooled to 0oC, filtered and washed with water (200 mL), ethanol (2 x 20 mL) and dry ether (3 x 20 mL) leaving a slightly pink solid. To a stirring solution of the pink solid [1-hydroxy-1,2-benziodoxol-3(1H)-one] (20g, 71 mmol, 1 equiv) in acetic anhydride (80 mL, 286 mmol, 4 equiv) was added TsOH.H2O (100 mg). The flask was equipped with a drying tube and was heated in an oil bath at 80 oC for 2 h. The resulting brown slurry was cooled to 0 oC in ice-water and filtered thruogh a fritted glass funnel. The resulting white solid was washed with anhydrous ether (5x50 mL) resulting in a white crystalline solid (15.2 g, 40 %)
Author Comments
This procedure has been succesfully carried out numerous times within the group on scales ranging from 5 g to 30 g of the produced D-M reagent. The literature claims that the first formed product is explosive but we have had no problems thus far. However, the preparation should only be carried out behind a blast shield. One problem with the first part of this procedure is that stirring becomes very difficult when addition of the potassium bromate is almost complete and hot pockets are produced in the mixture. This is prevented by the use of an overhead stirrer. The D-M reagent should be stored in a freezer in an amber bottle.
Data
1H nmr (300 MHz, CdCl3) 2.26 (3H, s), 7.71 (1H, dt, J=0.9, 7.8 Hz), 7.92 (1H, dt, J=1.5, 7.4 Hz), 8.00 (1H, d, J=8.1 Hz), 8.25 (1H, dd, J=1.2, 7.5 Hz)
Lead Reference
Dess, D.B.; Martin, J.C.; J. Org. Chem., 1983, 48, 4156; Ireland, R.E.; Liu, L.; J. Org. Chem., 1993, 58, 2899.
Other References
Greenbaum, F.R.; Am. J. Pharm, 1936, 17.
Keywords
Dess-Martin, Hyper-valent iodine, Oxidation
Comments
For a good example of a dess-martin oxidation, see SP 126.
By zac etheridge on August 23, 2001
See [[126|Page 126]] for the oxidation of allylic alcohols to enones using the DMP reagent
By Stephen Caddick on August 23, 2001
An nice alternative to the use of KBrO3/H2SO4 in the first step is oxone, see Frigerio, Marco; Santagostino, Marco; Sputore, Simona. A user-friendly entry to 2-iodoxybenzoic acid (IBX). J. Org. Chem. (1999), 64(12), 4537-4538.
By Craig Jamieson on March 13, 2002
oxone is better method to prepare the ibx. and ibx is converted to DMP easily in Ac2O and Cat.TsOH
By shy liu on September 15, 2006