Sandmeyer isonitrosoacetanilide isatin synthesis
SyntheticPage 464
DOI:
Submitted: August 10, 2010, published: August 10, 2010
Authors
Christopher Cooksey (rsc@chriscooksey.demon.co.uk)
A contribution from
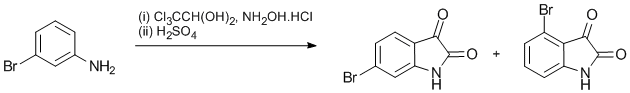
Chemicals
Chloral hydrate (Aldrich)
3-Bromoaniline (Aldrich)
Hydroxylamine hydrochloride (BDH)Procedure
A 5-litre flask was charged with (i) a solution of chloral hydrate (45 g, 0.27 mol) and Na2SO4.10H2O (320 g) in water (600 cm3), warmed 30 °C to dissolve the solids, (ii) 3-bromoaniline (43 g, 0.25 mol) dissolved with warming in water (150 cm3) and conc HCl (25 cm3) and (iii) a solution of hydroxylamine hydrochloride (55 g, 0.79 mol) in water (250 cm3). A thick white suspension formed. The mixture was heated. At 60 – 70 °C a thick paste formed. After heating for 2 h at 80 – 100 °C the mixture was cooled to 80 °C and filtered. The filtrate yielded more solid (2.4 g) after cooling. The pale brown product (3-bromoisonitrosoacetanilide) was washed by stirring with water (400 cm3) followed by filtration. Drying in air for 2 d gave the product (81.2 g). The expected yield was 61 g, so still wet.
With mechanical stirring, conc H2SO4 (200 cm3) was heated to 60 °C in a flask which was then removed from the heating mantle. 3-bromoisonitrosoacetanilide (15 g) was added over 20 m in portions so that the temperature remained between 60 and 65 °C. The mixture was then heated to 80 °C, then cooled to 70 °C and poured on to crushed ice (2.5 litres). After standing 1 h, the orange precipitate was filtered and washed with water (2 x 60 cm3), drying at 40 °C to give the mixture of 4-bromo- and 6-bromo-isatin as a pale orange powder (8.9 g).
Some of the bromoisatin mixture (10.5 g) was dissolved in hot (60 °C) NaOH solution (2M, 35 cm3) to give a dark brown solution. This solution was acidified with acetic acid (3.6 cm3) and the resulting orange-brown crystals filtered and washed with hot water to give 4-bromoisatin (5.7 g). The combined filtrates were warmed to 80 °C and conc HCl (5 cm3) added. After cooling overnight in a fridge, the bright orange crystals were filtered to give 6-bromoisatin (3.3 g).Author Comments
The 3-bromoisonitrosoacetanilide synthesis went well, probably in high yield, and was used directly for the second part. Holt et al 1958 report refluxing for 15 min gave a 55% yield of 3-bromoisonitrosoacetanilide.
The temperature of the sulfuric acid used in the second step is critical – too cold and no reaction occurs, too hot and decomposition occurs. Holt et al 1958 report a yield for 4-bromoisatin (46%) and 6-bromoisatin (21%). 7-Bromoisatin was prepared in the same way in 74% yield (lit: 29%).
A nice (Sandmeyer T, Helv Chim Acta, 1919, 2, 237) almost 19th century bucket chemistry synthesis.
An alternative separation of 4-bromo- and 6-bromo-isatin using high-speed counter-current chromatography (HSCCC) has been suggested: Almeida MR, Leitão GG, Silva BV, Barbosa JP, Pinto AC, J Braz Chem Soc, 2010, 21(4), 764-769; doi: 10.1590/S0103-50532010000400025.Data
4-bromoisatin:
δH (dmso-d6) 11.18s (1H), 7.44t (1H, 7.9), 7.20dd (1H, 0.8, 8.1), 6.87dd (1H, 7.7, 0.7)6-bromoisatin:
m.p. 268.1 °C (lit. Holt et al 1958, 270 °C)
δH (dmso-d6) 11.15s (1H), 7.42d (1H, 7.9), 7.24dd (1H, 8.4, 1.6), 7.06d (1.6)
δC (dmso-d6) 183.21s, 159.28s, 151.65s, 131.73s, 126.15d (169), 125.67dd (173, 5.0), 115.04dd (171, 5.3), 116.96sLead Reference
Clark RJH, Cooksey CJ, New J Chem, 1999, 3, 323-328; DOI: 10.1039/a808562e
Other References
Holt SJ, Sadler PW, Proc Roy Soc Lond B, 1958, 148(933), 481-494; PMID: 13542639; doi: 10.1098/rspb.1958.0040
(isomer separation) Sadler PW, J Org Chem, 1956, 21, 169-170: doi: 10.1021/jo01108a004
(isatin) Marvel CS, Hiers GS, Org Syn, 1925, 5, 71, Coll. Vol. 1, 1941, 327Keywords
bromoisatins, bromoisonitrosoacetanilide, Sandmeyer