Methyl ester hydrolysis
SyntheticPage 442
DOI:
Submitted: July 21, 2010, published: July 22, 2010
Authors
Christopher Cooksey (rsc@chriscooksey.demon.co.uk)
A contribution from
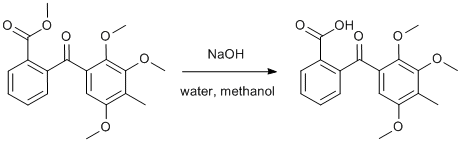
Chemicals
Methyl 2-(2,3,5-trimethoxy-4-methylbenzoyl)benzoate page 441
NaOHProcedure
Author Comments
Evaporation of the filtrate and refrigeration gave a further 257 mg of product. Some product (464 mg) was recrystallised from methanol (5 cm3) and water (15 cm3) to give white crystals (379 mg), mp. 149.3 ºC.
Raistrick H, Robinson R, Todd AR, J Chem Soc 1937, 80-88, doi: 10.1039/JR9370000080, obtained a product with a very different m.p. 205-208 ºC, from 2,3,6-trimethoxytoluene, phthalic anhydride, AlCl3 in CS2, perhaps the alternative isomer, 2-(2,4,5-trimethoxy-3-methyl-benzoyl)-benzoic acid.
A reaction using 28.2 g of starting material gave a 99% yield.
Using the same procedure, 2-(2,3,5-trimethoxy-benzoyl)-benzoic acid methyl ester was hydrolysed to give 71% of the corresponding acid.Data
Keywords
carboxylic acids, esters, hydrolysis