Arene bromomethylation
SyntheticPage 423
DOI:
Submitted: June 1, 2010, published: June 1, 2010
Authors
Suzanne Elizabeth Howson (s.e.howson@warwick.ac.uk)
A contribution from
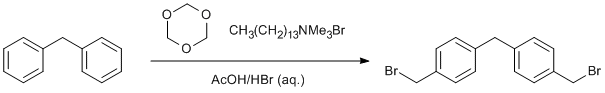
Chemicals
HBr (48% aq.)
Acetic Acid (glacial)
1,3,5-Trioxane
Tetradecyltrimethylammonium Bromide
Procedure
Diphenylmethane (5.00 g, 29.72 mmol, 1.0 eq.) was added to a solution of 48% aq. HBr (80 ml) and glacial acetic acid (21 ml). 1,3,5-trioxane (5.35 g, 59.44 mmol, 2.0 eq.) was then added to the mixture, followed by tetradecyltrimethylammonium bromide (0.16 g, 0.48 mmol, 0.016 eq.). The reaction was stirred at reflux (125°C) overnight (16 h). After this time the solution was cooled to ambient temperature, then to 0°C in an ice-water bath. The dark yellow solid was filtered off and washed with several portions of water. The solid was then dissolved in DCM (150 ml), washed with water (100 ml) before drying the DCM layer over Na2SO4. This was filtered and the solvent removed in vacuo leaving a light yellow solid, which was recrystallised from hot DCM leaving the pure product as a white solid. Yield = 2.63 g, 7.43 mmol, 25%.
Author Comments
Data
1H NMR (400 MHz, 298 K, CDCl3) 7.32 (4H, d, 3JHH = 8 Hz, Ph), 7.15 (4H, d, 3JHH = 8 Hz, Ph), 4.48 (4H, s, CH2Br), 3.96 (2H, s, CH2).
13C{1H} NMR (100 MHz, 298 K, CDCl3) 141.1 (Ph), 135.7 (Ph), 129.3 (Ph), 129.2 (Ph), 41.3 (CH2), 33.4 (CH2Br).
Lead Reference
Keywords
addition, alkyl/alkenyl/aryl halides, aromatics/arenes, bromomethylation