Radical bromination
SyntheticPage 414
DOI:
Submitted: May 27, 2010, published: May 27, 2010
Authors
David Fox (d.j.fox@warwick.ac.uk)
A contribution from
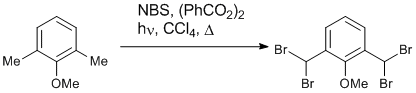
Chemicals
Procedure
2,6-Dimethylanisole (4.08 g, 30 mmol) was dissolved in CCl4 (250 ml) along with benzoyl peroxide (10 mg). N-Bromosuccinimide (21.36 g, 120 mmol) was added and the mixture was heated at reflux for 6 hours while being irradiated with a 250 W light-bulb. The reaction was allowed to cool and was then filtered to give a yellow solution. This solution was reduced in vacuo to give 2,6-bis(dibromomethyl)anisole as a pale yellow solid (13.42g, 99%). A sample was recrystallised from hexane for characterisation.
Data
mp 102-104 oC (ref 100-102 oC); δH (200MHz, CDCl3) 7.91 (2H, d, J 8.0, m-CH), 7.37 (1H, t, J 8.0, p-CH), 7.02 (2H, s, CHBr2), 3.96 (3H, s, OCH3).
Lead Reference
B. L. Feringa and O. J. Gelling, J. Am. Chem. Soc., 1990, 112, 7599-7604.
Keywords
alkyl/alkenyl/aryl halides, aromatics/arenes, radical, substitution