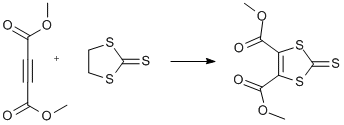
TTF I. Addition of dimethyl acetylenedicarboxylate to ethylene trithiocarbonate
SyntheticPage 277
DOI:
Submitted: June 5, 2008, published: June 5, 2008
Authors
Nikola Paul Chmel (n.chmel@warwick.ac.uk)
A contribution from
Chemicals
ethylene trithiocarbonate (Aldrich),
dimethyl acetylenedicarboxylate (Aldrich)
dimethyl acetylenedicarboxylate (Aldrich)
Procedure
Dimethyl acetylenedicarboxylate (10.4 g, 0.073 mol) and ethylene trithiocarbonate (10 g, 0.073 mol) were dissolved in toluene (25 ml). Mixture was refluxed overnight at 150°C. Product precipitated upon cooling (water/ice bath), filtered without washing, dried in vacuo (Yield 16.8g). Concentration of the filtrate gave second crop (Yield 2.4g) Overall yield (19.2g, 89%)
Author Comments
Has been performed several times with similar yields. Product used immediately in subsequent reaction.
Data
1H NMR (400MHz, Acetone-d6, 298K) 3.91 (s, CH3)
Lead Reference
L.R. Melby, H.D. Hartzler, W.A. Sheppard, J.Org. Chem. Vol. 39, No. 16, (1974) 2456-2458
Keywords
Comments
help
hello
i need the mechanisme of Addition of dimethyl acetylenedicarboxylate to ethylene trithiocarbonate.
By oussama on December 9, 2016
help
hello
i need the mechanisme of Addition of dimethyl acetylenedicarboxylate to ethylene trithiocarbonate.
By oussama on December 9, 2016