Nitrile reduction using Pd/C
SyntheticPage 262
DOI:
Submitted: April 13, 2007, published: April 16, 2007
Authors
Tim Snape (t_snape@hotmail.com)
A contribution from
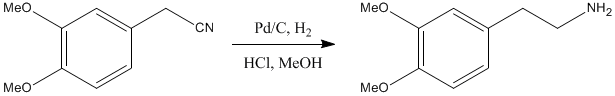
Chemicals
3,4-Dimethoxyphenylacetonitrile (Aldrich, 98%)
10% Palladium on carbon (Aldrich)
Solvents and conc. HCl (Analytical Reagent Grade)
10% Palladium on carbon (Aldrich)
Solvents and conc. HCl (Analytical Reagent Grade)
Procedure
3,4-Dimethoxyphenylacetonitrile (2 g, 11.3 mmol) was placed in a 1L single-neck round-bottomed flask containing a magnetic stirrer bar and was dissolved in MeOH (225 mL). Concentrated HCl (9.4 mL) and 10% Pd/C (1 g) were added and the flask was sealed with a new septum. The air was evacuated with a diaphragm pump through the septum using a needle. A balloon of hydrogen replaced the diaphragm pump and the evacuation process was repeated once more. The reaction was left to stir at room temperature for 2 hours. The reaction mixture was filtered through a pad of celite® in a sintered funnel under a blanket of nitrogen; CARE! DO NOT ALLOW Pd TO DRY OUT IN AIR. The celite was washed with MeOH (100 mL) and the solvents were removed under reduced pressure. The resulting pale brown oil was suspended in DCM (50 mL) and 2M NaOH (20 mL) added. The layers were separated and the organic layer washed washed with 2M NaOH (20 mL). The combined aqueous layers were extracted with DCM (20 mL), dried (MgSO4) and the solvent was removed under reduced pressure to yield the desired amine as an off-white solid (1.98 g, 97%) which was very pure by NMR.
Author Comments
The difficulty in sourcing the product 2-(3,4-dimethoxyphenyl)ethylamine from commercial suppliers led to the procedure being developed. Current literature methods are plagued with contamination by dimer and trimer formation, including those that claim not to produce these products (see lead ref.). Higher dilution and catalyst loading, along with the use of HCl to protonate the amine as it forms helps to prevent these by-products. CARE! must be taken when filtering off the palladium, especially on larger scale.
Data
1H NMR (300 MHz, CDCl3) 1.68 (2H, br. s), 2.70 (2H, t, J=6.8), 2.95 (2H, t, J=6.8), 3.85 (3H, s), 3.87 (3H, s), 6.72 (1H, s), 6.73 (1H, d, J=7.7), 6.80 (1H, d, J=7.7)
Lead Reference
K. Diker, M. Doe de Maindreville and J. Levy, Tetrahedron Lett, 1995, 2497-2500.
Keywords
Comments
Is there any effect of methoxy groups on yeild? if any effect of electoron withdrawing groups
By Veeresha Sharma P M on March 20, 2008
The difficulty in sourcing this material is because it comes under the UK Home Office Misuse of Drugs Act 1971 Schedule 1 paragraph 1 (d) as a controlled substance. Be aware that you should have a licence.
www.drugs.gov.uk/drugs-laws/licensing
By Colin Swinburne on June 30, 2008
In the USA, this compound is not regulated because even though it has positional isomer similarities to compounds convertable to abusable substances, the Federal Laws do not interfere with its sourcing...presently.
By Mark Bertinetti on November 4, 2008