Schiff-base condensation with pyridine carboxaldehyde
SyntheticPage 252
DOI:
Submitted: January 11, 2007, published: January 11, 2007
Authors
David Haddleton (d.m.haddleton@warwick.ac.uk)
A contribution from
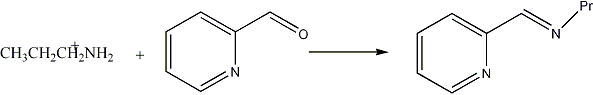
Chemicals
2-Pyridine carboxaldehyde (Aldrich)
Diethyl ether
Anhydrous magnesium sulphate
n-Propylamine (Aldrich)
Diethyl ether
Anhydrous magnesium sulphate
n-Propylamine (Aldrich)
Procedure
2-Pyridine carboxaldehyde (20 mL, 0.21 mol) and diethyl ether (20 mL) were added to a flask containing dried magnesium sulphate (BDH) (5 g). The flask was cooled to 0oC and n-propylamine (19 mL, 0.25 moles) was added slowly. The mixture was removed from the ice bath and stirred for two hours at 25oC prior to filtration. Diethyl ether was removed by rotary evaporation and the resulting yellow oil was purified by vacuum distillation.
The product was obtained as a straw-colored oil (bp. 43oC at 7 E 10-2 mbar). Yield = 98 %
Author Comments
Ligand for copper(I) for living polymerisation of acrylics. Works for many different amines - now used in an undergraduate laboratory.
Data
CHN analysis returned 73.1 % C, 8.2 % H, 18.7 % N, compared to theoretical 72.9 % C, 8.2 % H, 18.9 % N.
The 300 MHz 1H NMR spectrum (Bruker 300) in CDCl3 at ambient temperature shows aromatic absorptions from the pyridyl ring at 8.63 (d, 1H), 8.00 (d, 1H), 7.69 (t, 1H) and 7.27 (t, 1H) ppm from TMS. The proton alpha to the pyridyl ring returns a singlet at 8.39 ppm, while the protons of the propyl group are found at 3.64 (t, 2H alpha to N, J = 4.4 Hz), 1.76 (sx, 2H beta to N, J = 4.4 Hz), and 0.70 (t, 3H, J = 4.4 Hz) ppm.
The 75 MHz 13C NMR spectrum in CDCl3 at ambient temperature shows a C=N peak at 161.0 ppm, aromatic peaks at 154.1. 148.7, 135.8, 123.9 and 120.5 ppm, and peaks corresponding to the propyl group at 62.6, 22.3 and 11.3 ppm.
The IR spectrum (Bruker Vector 22 FTIR spectrometer equipped with a Golden Gate diamond attenuated total reflection (ATR) ) shows C-H stretches at 3054, 3009 and 2961-2834 cm-1, a C=N stretch at 1651 cm-1 and aromatic ring stretches at 1587, 1567, 1467 and 1436 cm-1.
The chemical ionization mass spectrum returned a major peak at m/z = 149 (M + H).
Lead Reference
D. M. Haddleton, D. J. Duncalf, A. M. Heming, D. Kukulj, and A. J. Shooter Macromolecules 31, 2016, (1998).
Other References
D. M. Haddleton, M. C. Crossman, B. H. Dana, D. J. Duncalf, A. M. Heming, D. Kukulj, and A. J. Shooter, Macromolecules 32, 2110 (1999).
Keywords
Comments
Here Diethyl Ether was used as solvent , would you please inform whether you tried using Ethanol or Dioxane , as these are assumed to be better solvents for the Schiff base synthesis
By Matloob Ahmad on October 6, 2007
Good!
By xp bao on December 26, 2007