Alkylation of boron trifluoride with pentafluorophenyl Grignard reagent; Tris(pentafluorophenyl)boron
SyntheticPage 215
DOI:
Submitted: October 20, 2003, published: November 5, 2003
Authors
Dr Simon Lancaster (S.Lancaster@uea.ac.uk)
A contribution from
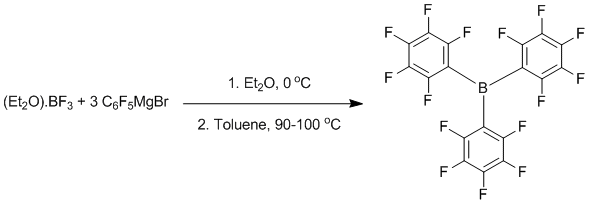
Chemicals
BrC6F5 (Fluorochem),
Mg turnings (Aldrich),
BF3.Et2O (Lancaster),
diethyl ether (dried over Na / benzophenone), toluene (dried over Na)
Mg turnings (Aldrich),
BF3.Et2O (Lancaster),
diethyl ether (dried over Na / benzophenone), toluene (dried over Na)
Procedure
Suspend magnesium turnings (7.2 g, 0.3 mol) in diethyl ether (500 mL) at room temperature. Add BrC6F5 (74.1g, 0.3 mol) dropwise until the solution develops a grey turbid appearance. Cool the reaction on an ice bath and adjust the dropping rate to ensure that the solution does not reflux. Stir for an additional hour to ensure complete reaction. The resulting solution will be dark brown-black in appearance. Dissolve BF3.Et2O (0.1 mol, 14.19 g) in toluene (200 mL) and cool to 0 ºC. Add the Grignard reagent solution via a cannula with vigorous stirring. After addition allow the resulting solution to warm to room temperature. Remove approximately 500 mL of solvent under reduced pressure. Fit a reflux condenser and heat the solution to (almost) 100 ºC on a boiling water bath for one hour. During this time glassy solids will precipitate from solution. Remove all volatiles under vacuum until a dry brown cake is obtained (colourless crystals of B(C6F5)3 may already be apparent). The product is isolated by extracting this cake with warm (45 ºC) hexanes (600 mL). As the hexane solution cools very fine feathery crystals will form. Cooling to -30 ºC will cause further crystallisation. The very fine nature of B(C6F5)3 crystals obtained in this fashion makes them difficult to separate by filtration from the mother liquor. One solution is to add 30 mL of diethyl ether to the collection flask before filtration, now instead of obtaining feathery crystals of B(C6F5)3, cubic crystals of (Et2O)B(C6F5)3 form, which can be easily separated by filtration. The yield depends upon the effort invested in the extraction process. We will typically extract the magnesium halides three times. For subsequent extractions we use the mother liquor obtained after crystallisation of the previous fraction. The best isolated yield obtained using this method was 70% Pure B(C6F5)3 can be obtained from (Et2O)B(C6F5)3 or the crude product described above by sublimation (<0.1 mmHg, 100 ºC).
Author Comments
The preparation should be performed using standard Schlenk techniques. Cooling the Grignard reagent preparation minimises the extent to which the solution darkens, which we associate with decomposition, it also avoids the need to use a reflux condenser in what is a strongly exothermic reaction. It is necessary to remove the diethyl ether under reduced pressure before heating the reaction or sufficiently high reaction temperatures will not be obtained. The use of a boiling water bath is a device to obtain the optimum temperature. If the temperature is not high enough complete reaction will not occur and the product will be contaminated with (Et2O)B(C6F5)2F, which decomposes to give the oil B(C6F5)2OEt during sublimation. If the reaction is heated above 100 ºC the yield is significantly reduced, presumably due to decomposition. B(C6F5)3 is thermally stable but highly hygroscopic. We find it convenient to store samples long-term as the adduct, (Et2O)B(C6F5)3 and sublime as required.
Data
Note: The ether adduct partially dissociates in CDCl3 or C6D6 solution. The data presented here is for the ether-free material. 11B NMR (293 K, d6-benzene): 63 19F NMR (293 K, d6-benzene): -130 (o-F), -144 (p-F), -161 (m-F)
Lead Reference
1. J. L. W. Pohlmann and F. E. Brinckman, Z. Naturforsch, 1965, 20b, 5.
Other References
2. A. G. Massey, A. J. Park and F. G. A. Stone, Proc. Chem. Soc., 1963, 212; A. G. Massey and A. J. Park, J. Organomet. Chem., 1964, 2, 245.
Keywords
215, addition, boron, pentafluorophenyl, sulphides, thermal
Comments
excellent procedure
Following some difficulty preparing pure samples of B(C6F5)3 for some of my coworkers, I followed this procedure on approx 50g scale (BrC6F5) and it worked brilliantly first time. A really nice, clear, prep.
By Michael Cowley on September 9, 2013
excellent procedure
I distilled the BF3 etherate and BrPhF5 (from P2O5) right prior to synthesis, and used alumina-dried solvents which were stored for 24 h over freshly activated 3A molecular sieves.
The only cumbersome is if you are impatient with the final extraction out of Mg halides and do it at higher temperature - if performed at, say, 60 degrees, and filtered via canula, it quite rapidly gets blocked, as the solubility drastically drops from 60 degrees to RT.
By Eduards Bakis on June 9, 2017