Generation of a Highly Basic and Nucleophilic Organolithium
SyntheticPage 195
DOI:
Submitted: March 25, 2002, published: March 26, 2002
Authors
Matthew Stent (matthew.stent@chem.ox.ac.uk)
A contribution from
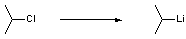
Chemicals
Lithium granules (high sodium, Aldrich)
Isopropyl chloride (Aldrich, freshly distilled from MgSO4, boiling point: 35 °C)
Petrol (boiling point 40 - 60 °C, freshly distilled from CaH2)
Isopropyl chloride (Aldrich, freshly distilled from MgSO4, boiling point: 35 °C)
Petrol (boiling point 40 - 60 °C, freshly distilled from CaH2)
Procedure
Caution:
Isopropyllithium is highly flammable, pyrophoric and reacts violently with water releasing highly flammable gas. All operations should be conducted with oven dried glassware which has been allowed to cool under an atmosphere of argon.
Lithium (12.0 g, 1.73 mol, 3 eq.) is suspended in petrol (145 cm3) in a 500 cm3 round bottom flask fitted with a pressure equalising dropping funnel and a condenser. The temperature of the vigorously stirred suspension is raised to reflux for 1.5 - 2 h, during which time the solvent becomes discoloured as oxide is cleaned from the surface of the lithium. The external heat source is then removed and approximately 2 cm3 of isopropyl chloride (total 53 cm3, 576 mmol) is added. An exothermic reaction initiates, and the remainder of the isopropyl chloride is added dropwise over 1 - 1.5 h at such a rate that reflux is maintained throughout. Following complete addition the purple solution continues to reflux under the reaction’s exotherm for approximately 1 h. External heat is then reapplied such that the reaction is at reflux for a further hour.
The reaction is then allowed to cool to room temperature under a fast stream of argon. The condenser and dropping funnel are quickly replaced with stoppers and a filter stick (approximately 5 cm in diameter) fitted into the third neck. Filtration under argon of the suspension through the glass sinter into a 250 cm3 round bottom flask gives a colourless solution of isopropyllithium in petrol, which is titrated (against 2,2,2’-trimethylpropionanilide)1 as 1.3 - 1.6 M.
Author Comments
This procedure has been conducted numerous times in our laboratory, usually without incident. If conducted on the stated scale the concentration generally varies only within the given range, however, reducing the scale of the reaction by even 25% appears to have a detrimental effect. The lifetime of the isopropyllithium is obviously related to the use it receives, we would expect a solution from which a few mL’s are removed daily to last (with some reduction in concentration) for two - three weeks. Within the first 48 h of preparation a white precipitate usually begins to form, but ‘sticks’ to the surface of the glass and can easily be avoided when syringing. Once the solution has become orange in colour (three - four weeks) it should be discarded.
The procedure is a fairly straight forward organometallic preparation, with only the filtration being tricky - a second pair of hands generally proves useful. Our filter stick has ground glass taps either side of the sinter, allowing a fast stream of argon to be applied to the ‘reaction side’ whilst a slight vacuum is applied to the ‘collection side’ to draw the solution through. Following filtration the filter stick should be quickly exchanged for a ground glass tap and the solution of isopropyllithium stored in the fridge.
The use of granular lithium, though requiring the initial cleaning time, is beneficial as it is easily handled. Lithium powder has been used, with similar results, however requires a glove bag to be handled safely. Following filtration excess lithium can be washed back into the reaction flask with petrol and quenched by cautious addition of isopropyl alcohol the vigorously stirred suspension cooled at 0 °C.
Following use, and usual cleaning, (or before anticipated use) the sinter should be washed with a substantial quantity of conc. HCl, water and finally acetone, before oven drying for at least 24 h. Failure to rigorously clean the sinter in this way usually results in an orange solution of low concentration and short life.
Other References
1. J. Suffert, J. Org. Chem., 1989, 54, 509 – 510.