Epoxidation of an alpha-keto ester
SyntheticPage 194
DOI:
Submitted: March 19, 2002, published: March 19, 2002
Authors
Dr Russell Cox (r.j.cox@bris.ac.uk)
A contribution from
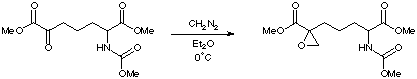
Chemicals
Diethyl ether,
Diazomethane,
Alpha-keto ester.
Diazomethane,
Alpha-keto ester.
Procedure
Dimethyl (2RS)-2-(N-(methyloxycarbonyl)amino)-6-oxopimelate (300mg, 1.04mmol) was treated with a solution of CH2N2 in diethyl ether at 0oC for 1h. Solvent was removed in vacuo and the residue purified by flash chromatography (50% EtOAc in hexane, Rf 0.5; 10% CH3CN in CH2Cl2 was also effective, Rf 0.27) to give Dimethyl (2RS, 6RS)-2-(N-(methyloxycarbonyl)amino)-6-epoxymethanopimelate as a colourless oil (273.4mg, 91%).
Author Comments
The key points are:
1. keep the reaction cooled at 0oC - warming gives methylene insertion compounds.
2. The ethereal solution of diazomethane is made according to the procedure of Vogel (B. S. Furniss, A. J. Hannaford, P. W. G. Smith and A. R. Tatchell, "Vogels Textbook of Organic Chemistry" 5th Ed. Longman, New York, 1989, pp 430-433.)
3. Diazomethane is reputedely toxic, carcinogenic and explosive - the ethereal solution is very much safer than neat diazomethane, but care must be taken to avoid ground glass joints and sharp edges of broken glass to avoid the explosion risk. Use of a blast shield is advised.
4. Small quantities of excess diazomethane/ether ( ca 10ml) are harmlessly destroyed by rotavapping off - for larger quantities neutralise by addidition of glacial acetic acid in ether.
Data
vmax (thin film)/cm-1 3605, 3510, 3369, 2999, 2955, 1736, 1530, 1440; 1H(300MHz, CDCl3, two diastereomers) 5.45 (1H, d, 3J 7.9, NH), 4.35 (1H, m, CH), 3.76, 3.75, 3.74, 3.72, 3.71, 3.68 (9H, 3 x OMe), 3.05 (1H, m, CH), 2.80 (1H, m, CH), 2.17 (1H, m, CH), 1.88 (1H, m, CH), 1.70 (2H, m, CH + CH), 1.55 (2H, m, CH + CH); 13C(75.5MHz, CDCl3, two diastereomers) 172.9, 171.8, 170.6, 170.0, 156.7, 156.6, 58.0, 57.9, 56.63, 56.60, 53.7, 53.4, 52.6, 52.4, 52.3, 52.1, 46.1, 45.6, 32.3, 30.8, 30.62, 29.4, 21.4, 20.8; m/z (CI, CH4) 290 (MH)+, 5%; 258 (M-MeO)+, 10%; Calcd. for C12H20NO7 (MH)+ 290.12398, Found 290.12367.
Lead Reference
See R. J. Cox, J. A. Durston and D. A. Roper, J. Chem. Soc. Perkin Trans 1., 2002, in press.
Other References
Vogel : B. S. Furniss, A. J. Hannaford, P. W. G. Smith and A. R. Tatchell, "Vogels Textbook of Organic Chemistry" 5th Ed. Longman, New York, 1989, pp 430-433.