Formation of imides from anhydrides
SyntheticPage 185
DOI:
Submitted: March 7, 2002, published: March 7, 2002
Authors
Ashley Parker (ashley.parker@bristol.ac.uk)
A contribution from
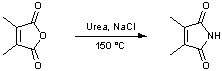
Chemicals
2,3-Dimethylmaleic anhydride (Lancaster), urea (Aldrich), sodium chloride
Procedure
A mixture of 2,3-dimethylmaleic anhydride (5 g, 39.65 mmol), urea (2.38 g, 39.65 mmol, 1 equiv.) and sodium chloride (8.11 g, 138.8 mmol, 3.5 equiv.) are ground by pestle and mortar to give a uniform mixture. The mixture is transferred to a flask fitted with an air condenser and is heated with stirring at 150 °C until the organic components are seen to fuse. Heating is continued for 30 m with localised heating of the apparatus by hot-air gun where vapours are seen to condense. Boiling water (20 cm3) is then added. The cooled mixture is diluted with water (30 cm3) and extracted (EtOAc, 4 X 50 cm3). The combined organic layers are washed (NaCl sat. aq., 30 cm3), dried (MgSO4) and concentrated in vacuo. The residue is redissolved in toluene (20 cm3) and concentrated in vacuo to give an orange solid. Purification by dry column vacuum chromatography2 (eluting with 0-100 % EtOAc in 40-60 petroleum ether with increments of 10 %) gives 2,3-dimethylmaleimide as a colourless crystalline solid (3.921 g, 79 %).
Author Comments
This and other 2,3-disubstituted maleimides, which are commonly used starting materials in our group, can be conveniently prepared on a multigram scale from the corresponding anhydrides. This simple procedure represents a practical improvement over literature methods as the use of ammonia gas or reaction solvent is not necessary. Purification by dry column vacuum chromatography allows excellent product recovery on a large scale compared to recrystallisation.
Data
1H NMR (270 MHz, CDCl3) 1.97 (6H, s, 2 X CH3).
Lead Reference
1Y. L. Chow, Y. M. A. Naguib, J. Chem. Soc. Perkin Trans. 1, 1984, 1165.
2D. S. Pedersen, C. Rosenbohm, Synthesis, 2001, 2431.