Formation of diisopropylphosphoramidous dichloride by monoamination of phosphorus trichloride
SyntheticPage 184
DOI:
Submitted: February 21, 2002, published: February 21, 2002
Authors
João M. Navarro y Rosa (jmr05340@students.si.fct.unl.pt)
A contribution from
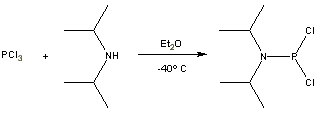
Chemicals
Diisopropylamine (distilled from CaH2)
PCl3 (Merck)
Ethyl ether (distilled from Na/benzophenone)
PCl3 (Merck)
Ethyl ether (distilled from Na/benzophenone)
Procedure
To a solution of phosphorus trichloride (8,7 ml, 0.10 mol) in dry ether (100 ml) at –40º C, under dry argon atmosphere, diisopropylamine (26,2 ml, 0.20 mol) was added over 1h, under vigorous stirring. During this step, temperature should not be allowed to rise, and the diisopropylamine hydrochloride precipitate that forms must not be allowed to clog the reaction mixture (more dry ether may be added, if necessary). The reaction temperature was then allowed to rise to 0º C (ice bath) and left overnight, after which the precipitate was removed via canula filtering, under dry inert atmosphere (the precipitate should be washed with dry ether, to remove most of the product). The resulting solution was concentrated through distillation under dry atmosphere until most ether was removed, and the crude product was distilled in vacuo (85º C, 1 mmHg), yielding the title product as a colourless liquid (16 g, 80%).
Author Comments
This reaction has also been performed by other worker in our lab using diethylamine, with similar results. It works well provided that rigorous exclusion of moisture is maintained and clogging is avoided. When these conditions were not met, we observed reduced yields due to hydrolysis and/or polyamination. The title product was used as a starting material for further reactions, so this reaction was performed about 5 times in multigram scale (5-20g) and the product kept in storage (we do not synthesise this compound routinely). The product is relatively stable to oxidation by O2, but very sensitive to moisture. It should be stored at low temperature (crystallizes). A sample has been kept for 4 years at 4º C with little decomposition (sublimation was observed).
Data
1H nmr (400 MHz, CDCl3) 3.92 (2H, hept, J = 6.5 Hz), 1.27 (12H, d, 6,8 Hz).
31P nmr (160 MHz, CDCl3) 170.
Lead Reference
Perich, J.; Johns, R. B., Synthesis, 1988, 142-144.