Benzylic bromination of 4-bromo-3,5-dimethylanisole using N-Bromosuccinimide
SyntheticPage 159
DOI:
Submitted: August 31, 2001, published: August 31, 2001
Authors
Mark Birri (mark_birri@hotmail.com)
A contribution from
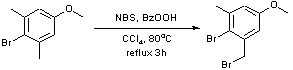
Chemicals
4-bromo-3,5-dimethylanisole (see Synthetic Page X),
N-Bromosuccinimide (NBS) (Avocado, recrystallised),
Benzyl peroxide (Aldrich),
Carbon tetrachloride.
N-Bromosuccinimide (NBS) (Avocado, recrystallised),
Benzyl peroxide (Aldrich),
Carbon tetrachloride.
Procedure
A solution under N2 of 4-bromo-3,5-dimethylanisole (39.5g, 183.8mmol), N-bromosuccinimide (32.7g, 183.8mmol), benzyl peroxide (2.23g, 9.19mmol) in CCl4 was heated at reflux at 80oC for 3h. After cooling to r.t, the light yellow solution filtered and concentrated to give an orange oil which precipitated a yellow/white solid. Recrystallisation from 9:1 hexane/ethyl acetate yielded white crystals of 3-bromomethyl-4-bromo-5-methylanisole (17.5g, 32.4%).
Author Comments
A simple and effective reaction, however I am unsure about the percentage yield. This reaction has been carried out on this scale by other members of the group, usually yielding around 25g of product i.e. 50% yield.
Data
1H nmr (300MHz, CDCl3) 2.41 (3H, s), 3.80 (3H, s), 4.61 (2H, s), 6.77 (1H, d, J=3Hz), 6.86 (1H, d, J=3Hz)
Lead Reference
Rucker, M. Brueckner, R. Synlett, 1997, 1187-1189
Keywords
Comments
Note: The material referred to as "benzyl peroxide" is a misnomer (though it has been used in the popular literature). The actual initiator is dibenzoyl peroxide also reffered to as benzoyl peroxide.
By Leonard Wojcik on January 20, 2006
NBS is excellent reagent for the selective Benzylic bromination , Its nice that you got the desired product. Did you note the formation of 3,5-dibromomethyl-4-bromo anisole. As it seams that its formation should also occur though the reactants are in 1:1 mole equivalent.
By Matloob Ahmad on July 25, 2008