N-tosyliminobenzyliodinane - PhINTs from Iodobenzene diacetate
SyntheticPage 123
DOI:
Submitted: August 23, 2001, published: August 23, 2001
Authors
Kevin Gillespie (kevin.gillespie@warwick.ac.uk)
A contribution from
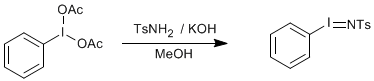
Chemicals
p-Toluenesulphonamide (Aldrich)
Potassium Hydroxide
Iodobenzene Diacetate (Aldrich)
Methanol
Diethyl Ether
Potassium Hydroxide
Iodobenzene Diacetate (Aldrich)
Methanol
Diethyl Ether
Procedure
p-Toluenesulphonamide (5.13 g), potassium hydroxide (4.20 g) and methanol (120 ml) were stirred in a conical flask in an ice bath, ensuring the reaction mixture was below 10oC. (It's not essential to have a solution, a suspension is more likely at this temp). Iodobenzene diacetate (9.60 g) was added to the stirred mixture and the resulting yellow coloured solution was stirred at room temperature for 3.5 h. The reaction mixture was poured into a large excess of iced water and stirred for 1 h. A yellow coloured solid precipitated on standing overnight. (It's important to allow the solid to stand as the particle size appears to increase giving higher yield on filtration). The light yellow solid was isolated by filtration and dried with a flow of air through through the buchner funnel. Several portions of ether, in which the product is insoluble were used to wash away any iodobenzene present. The yellow solid was dissolved in a minimum of boiling methanol. (It is important to keep this volume low in order to achieve a higher yield, 50-100 ml MeOH). The solution was placed in a freezer overnight whereupon an off-white solid was recovered via filtration. Yield = 6.70-7.80 g, 60-70%.
Author Comments
The yield of PhINTs from this reaction is never very high but it's important to achieve a high level of purity. This is difficult to determine due to the insolubility of the product. This also makes the material difficult to crystallise but sufficient heating will get the material into methanol. Dont be alarmed by the resulting brown colour of the methanolic solution. The solid will come out pale.
Data
1H NMR (d6-DMSO): d 7.73-7.16 (m, 9H, Ph), 2.37 (s, 3H, Me). 13C DEPT-NMR (d6-DMSO): d 142.50 (Ts), 142.20 (Ts), 141.58 (Ts), 137.46 (Ph), 131.02 (Ph), 129.65 (Ts), 129.59 (Ts), 128.06 (Ph), 126.09 (Ts), 125.98 (Ts), 95.23 (Ph), 21.29 (Me). EA for C13H12NO2SI. Calculated % C, 41.84; H, 3.24; N, 3.75. Found % C, 41.25; H, 3.22; N, 3.54.
Lead Reference
a) Yamada, Y; Yamamoto, T; Okawara, M Chem. Letts, 1975, 361-362. b) Besenyei, G; Nemeth, S; Simandi, L.I. Tetrahedron Lett.1993, 34, 6105-6106.
Keywords
123, Aziridination, Iodobenzene diacetate, nitrenes, PhINTs
Comments
CIF file?
Are there crystal structures available for the reactant and product? If so, what are the identifiers?
By Egon Willighagen on March 25, 2011
This is a notoriously insoluble compound of good utility but essentially unknown structure to my knowledge. Some analogues are known which are better characterised, but you will need to look for those by the usual means.
By Peter Scott on March 25, 2011
H-NMR incorrect (corrected in message)
The 3H singlet actually shows up around 2.3 (as reported by Yamada) I obtained a value of 2.27 (not 2.37 as mentioned in the Data above). Along with that, the 9 aromatic protons show up in the 7.69-7.05 range in d6-DMSO.
By Francisco S on October 10, 2015