Amidation of zirconium tetrachloride with dimethylamidolithium
SyntheticPage 113
DOI:
Submitted: August 21, 2001, published: August 21, 2001
Authors
Colin Morton (c.morton@warwick.ac.uk)
A contribution from
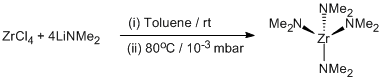
Chemicals
toluene (distilled from sodium)
lithium dimethylamide (Aldrich)
zirconium tetrachloride (Aldrich)
lithium dimethylamide (Aldrich)
zirconium tetrachloride (Aldrich)
Procedure
Toluene (80 ml) was added to a mixture of [Li(NMe2)] (3.7 g, 0.068 mol) and ZrCl4 (4.0 g, 0.017 mol) at room temperature. After stirring overnight, the solvent was removed in vacuo and the yellow residue was sublimed at 80°C and 10-3 mbar giving an off-white crystalline solid. Yield = 83%, 3.7g.
Author Comments
Standard schlenk line procedures are used. The sublimation was carried out using schlenk-line vacuum.
We have carried out this procedure many times.
Data
1H NMR (293 K, d6-benzene) 3.05 (s, 24H, NMe2).
Lead Reference
D. C. Bradley and I. M. Thomas, J. Chem. Soc., 1960, 3857.
Keywords
Comments
When adding the toluene to the amide, it can help to keep the mixture cool (acetone/dry ice) until there is sufficient solvent to move all of the solid. Otherwise a lot of heat can be generated causing a blackening of the solution.
By Edward Crust on August 22, 2001
Zirconium tetrachloride is used as supplied from Aldrich.
By Colin Morton on August 24, 2001
This compound is no longer comercially available, and in fact recently we have found that the commercial material has been of poorer quality thus reducing the yield of zirconium amide. We have however recently synthesised LiNMe2 from lithium butyl and dimethylamine (gas). This prep needs to be conducted with care and our first attempt was troubled with stirring problems, but we did isolate a excellent yield of pure white LiNMe2 - the commercial material was grey and getting greyer! My group will report this prep soon.
By Peter Scott on March 20, 2010
The synthesis is now published and a link has been added in "Chemicals Used" above
By Peter Scott on June 9, 2010
Insitu prep
Hi!
Can I do an insitu preparation of tetrakis(dimethylamido)zirconium? What we plan is to make LiNMe2 in Toluene(same process but use Toluene instead of pentane as mentioned) and in the same suspension add ZrCl4 at low temperature, without isolating the LiNMe2.
Regards,
Soumen
By Soumen Mukherjee on June 11, 2014
Not sure what you mean by "can I do". You can do it, but it is very unlikely to work so well. The sublimation will probably produce oily material alongside the amide which will then fail to crystallise. If you go ahead with this I would suggest you use an excess of the amine so that all the BuLi is used up, then evaporate to remove the excess amine. We published a detailed prep of LiNMe2 so that you do not have to cut corners like this. You need to do a full and complete risk assessment.
By Peter Scott on June 11, 2014