Deprotection of trimethylsilyl group of an alkyne
SyntheticPage 100
DOI:
Submitted: August 17, 2001, published: August 17, 2001
Authors
Marco Soscia (m.g.soscia@sussex.ac.uk)
A contribution from
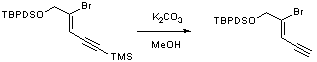
Chemicals
K2CO3 (aldrich anhydrous)
MeOH (distilled over CaH2)
MeOH (distilled over CaH2)
Procedure
To a solution of TMS-alkyne (14.86g, 31.52mmol) in methanol (250ml) was added potassium carbonate (0.54g, 3.9mmol) and the mixture stirred for 2hrs at room temperature under N2. The reaction mixture was then concentrated in vacuo, and the residue diluted with ether, washed with water, brine and dried over MgSO4, filtered and concentrated in vacuo. Purification by flash chromatography eluting with 25:1 petrol/ether yielded the title copmound as an orange solid (10.26g, 82%)
Author Comments
This transformation has been carried out numerous times in the lab on various substrates. Although in this example no problems were identified, in other examples serious reductuion in yield was noted when the reaction was left for longer periods.
Data
1H: 7.58 (m, 4H), 7.35 (m, 6H), 6.45 (s, 1H), 4.25 (s, 2H), 3.23 (s, 1H), 1.00 (s, 9H)
Lead Reference
S.Caddick, V.M.Delisser, V.E.Doyle, S.Khan, Tetrahedron, 1999, 2737-2754